What is Polyethylene Terephthalate (PET)?
PET plastic is an abbreviation for Polyethylene terephthalate, commonly referred to as PET or PETP. It mainly includes polyethylene terephthalate (PET) and polybutylene terephthalate (PBT).
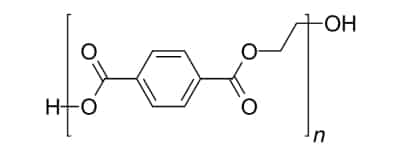
Part 1: Basic Knowledge of PET
The molecular structure of PET plastic is highly symmetrical and has a certain degree of crystallinity, giving it excellent film-forming and molding properties. PET plastic possesses good optical performance and weather resistance, and amorphous PET plastic has excellent optical transparency.
PET has good creep resistance, fatigue resistance, wear resistance, and dimensional stability, with low abrasion and high hardness. It also has the highest toughness among thermoplastic plastics, with good electrical insulation properties that are minimally affected by temperature, though it has poor corona resistance. It is non-toxic, weather-resistant, chemically stable, with low water absorption, resistant to weak acids and organic solvents, but not resistant to hot water immersion or alkalis.
PET resin appears as a milky white semi-transparent or colorless transparent material with a relative density of 1.38 and a light transmittance of 90%. PET is considered a medium-barrier material, with an oxygen transmission rate of 50-90 cm³•mm/(m²•d•MPa) and a carbon dioxide transmission rate of 180 cm³•mm/(m²•d•MPa). Its water absorption rate is 0.6%, indicating relatively high water absorption.
PET film has very high tensile strength, comparable to aluminum foil, nine times that of HDPE film, and three times that of PC and PA films. Reinforced PET has low creep, excellent fatigue resistance (better than reinforced PC and PA), good wear resistance, and good friction resistance. The mechanical properties of PET are less affected by temperature.
Pure PET has low heat resistance, but this significantly improves after reinforcement, with mechanical properties at 180°C better than PF laminate, making it one of the best heat-resistant thermoplastic engineering plastics. PET has good heat-aging resistance, with a brittle temperature of -70°C and retains some toughness at -30°C. PET is not easy to burn, with a yellow flame and dripping.
Although PET is a polar polymer, its electrical insulation is excellent, even at high frequencies. However, its corona resistance is poor, making it unsuitable for high-voltage insulation; its electrical insulation is affected by temperature and humidity, with humidity having a greater impact.
PET contains ester bonds and is not resistant to water, acids, and alkalis under high temperature and steam conditions. PET is stable to organic solvents such as acetone, benzene, toluene, trichloroethane, carbon tetrachloride, and oils. It also has high resistance to some oxidizing agents like hydrogen peroxide, sodium hypochlorite, and potassium dichromate. PET has excellent weather resistance and can be used outdoors for extended periods.
Part 2: Applications of PET
PET can be divided into two major categories based on its usage: fiber and non-fiber. The former is used to produce polyester staple fibers and polyester filaments, serving as raw material for polyester fiber companies to process fibers and related products (polyester being the most produced type of synthetic fiber). The latter includes films, containers, and engineering plastics.
1. Fiber Applications
In the early stages of development, PET was primarily used to produce synthetic fibers (accounting for about 70% of PET consumption). PET is also used to manufacture insulating materials, magnetic tape substrates, film or photographic film bases, and vacuum packaging. Another major non-fiber application of PET is the production of hollow containers for beverages, food, etc.
Polyester, known for its high fiber strength and good wearing performance, is currently the highest-produced synthetic fiber. Staple fibers can be blended with cotton, wool, or linen to make textiles for clothing or home furnishings, while filaments can be used for clothing or industrial purposes, such as filter cloth, tire cord fabric, parachutes, conveyor belts, and safety belts.
2. Non-Fiber Applications
Apart from fibers, PET is mainly used in three categories: films and sheets, bottles, and engineering plastics.
2.1 Films and Sheets
Mainly used for packaging materials such as food, pharmaceuticals, and non-toxic, sterile sanitary packaging; high-end packaging for textiles, precision instruments, and electronic components; substrates for audio tapes, video tapes, photographic film, movie film, disks, CDs, and magnetic cards; capacitor films, flexible printed circuit boards, and membrane switches.
PET films feature transparency, oil resistance, aroma retention, hygiene reliability, and a wide temperature range of use (suitable for both high-temperature sterilization and frozen packaging). However, they have poor heat-sealing properties and must be combined with other films (heat-sealing layers), and their relatively higher price compared to general plastic films limits their packaging applications.
2.2 Bottles
Since PET can easily obtain a nearly amorphous, highly transparent, and easy-to-stretch state through rapid cooling, PET can be used to produce biaxially oriented packaging films or high-strength, highly transparent stretch blow-molded bottles from amorphous preforms. PET can also be directly extruded or blow-molded into non-stretch hollow containers. PET bottles have high transparency and good barrier properties, making them suitable for use in fresh-keeping packaging materials, such as for beer, liquor, carbonated drinks, edible oil, food, condiments, pharmaceuticals, cosmetics, and health products.
2.3 Engineering Plastics
Reinforced PET varieties are mainly used in the following areas:
- Electronics and electrical: Connectors, coil winding tubes, integrated circuit housings, capacitor housings, transformer housings, TV parts, tuners, switches, timer housings, and relays.
- Automotive parts: Distribution board covers, valves, exhaust parts, distributor caps, small electric motor housings, structural components such as mirror housings, and electrical components such as headlamp reflectors.
- Mechanical parts: Gears, cams, pump housings, pulleys, motor frames, clock parts, motor housings, electrical connectors, relays, switches, microwave oven internal parts, etc.
- Industrial applications: Pump housings, manual tools, zipper materials. PET is the third-generation zipper material after PA and POM and can be used in both wide and narrow specifications.
Part 3: Production of PET
PET production varies based on the method used to produce its monomer, bis(2-hydroxyethyl) terephthalate (BHET). Currently, there are three main methods for producing BHET, namely transesterification, direct esterification, and ethylene oxide addition, forming three major polyester production processes.
1. Transesterification Method (DMT Method)
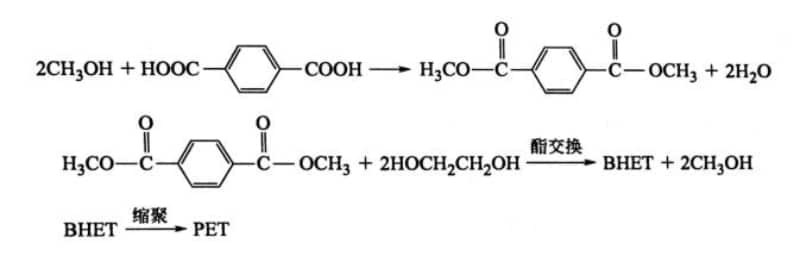
In this process, dimethyl terephthalate (DMT) reacts with ethylene glycol (EG) in a transesterification reaction, followed by polycondensation to form PET. In the early stages, the purity of the PTA monomer was not high, and it was difficult to purify, making it impossible to produce quality PET through direct polycondensation. Thus, impure TPA was first reacted with methanol to form dimethyl terephthalate (DMT), which is easier to purify. High-purity DMT (≥99.9%) is then used to react with EG in a transesterification reaction to produce BHET, which is subsequently polymerized into PET.
2. Direct Esterification Method (PTA Method)
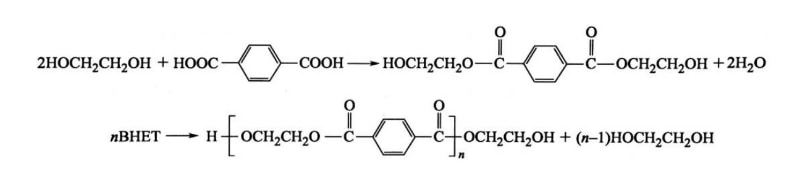
In this method, high-purity terephthalic acid (PTA) or medium-purity terephthalic acid (MTA) reacts directly with ethylene glycol (EG) to form polyester through esterification and polycondensation. This method developed rapidly after Amoco successfully purified crude terephthalic acid in 1965, and PET production expanded accordingly.
Using PTA as a raw material, PET polymer production involves two main reactions: first, PTA reacts with EG in an esterification reaction to produce bis(2-hydroxyethyl) terephthalate (BHET); second, BHET undergoes polycondensation in the presence of a catalyst to form PET.
Due to several advantages over the DMT method—such as lower raw material consumption, a smaller EG recovery system, no methanol byproduct, safer production, shorter process flow, lower capital investment, lower utility and production costs, smoother reaction rates, and more stable production control—most of the world’s PET production capacity now uses the PTA method.
3. Ethylene Oxide Addition Method (EO Method)
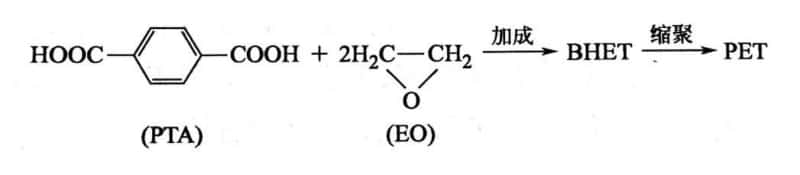
Since ethylene glycol is made from ethylene oxide (EO), PET can also be produced by directly reacting EO with PTA to obtain BHET, followed by polycondensation to form PET. This method is called the ethylene oxide addition method.
This process eliminates the step of producing ethylene glycol from EO, thereby reducing costs and speeding up the reaction, making it superior to the direct polycondensation method. However, since EO easily undergoes ring-opening to form polyethers and generates significant reaction heat (about 100 kJ/mol), and EO is a gas at room temperature that easily decomposes thermally, making transportation and storage difficult, this method has not yet been widely adopted.
Part 4: Modification of PET
1. Filling Modification of PET
Filling modification is one of the most direct and effective ways to comprehensively enhance material properties by using inorganic components that are completely different from the polymer matrix.
2. Nanoparticle Modified PET
Currently, research on nanoparticle-modified PET composites is quite mature. Ke et al. used layered clay to modify PET and obtained PET/clay nanocomposites through intercalation polymerization. The results showed that when the clay content was 5 wt%, the heat distortion temperature of the composite material increased by about 20°C to 50°C compared to pure PET, and the modulus of the composite doubled compared to PET.
3. Glass Fiber Modified PET
Compared to nanoparticles, micron-sized glass fiber (GF) has outstanding advantages in cost and controllability, making it widely used in filling modification of polymer materials.
4. Blending Modification of PET
Two or more polymers, including PET, are blended in appropriate proportions under specific temperature and shear stress conditions to form polymer alloys or blends with new properties. The compatibility between polymers is the key to the preparation of such polymers.
5. Polyolefin Modified PET
PET and PE have significant differences in chemical structure and are incompatible. Simple binary blending of the two shows that to improve the impact resistance of PET through polymer blending, compatibilization is necessary to enhance compatibility. In HDPE/PET blends, the impact strength improves when EVA and EAA are added.
When PET is blended with PP, the resulting alloy combines the advantages of both, improving properties, such as PET enhancing the heat resistance of PP, and PP reducing the sensitivity of PET to moisture. Without compatibilizers, PET/PP blends exhibit weak interfacial bonding and poor mechanical properties.
PET/PS is also an incompatible system, requiring a compatibilizer to achieve blending compatibility. Research has shown that adding styrene and glycidyl methacrylate copolymer P(S-GMA) as a reactive compatibilizer to the PET/PS blend system results in good interfacial bonding and improved mechanical properties in the PET/PS/P(S-GMA) blend system.
6. Polyester Modified PET
PBT is a new type of engineering plastic that developed rapidly in the 1970s. It has better mechanical properties than PET, as well as good toughness and moldability, but it has poorer heat resistance and flowability compared to PET and is more expensive. According to Teijin Corporation, adding 0.5% talc as a nucleating agent to the blend of PET and PBT results in a blend with good impact resistance and low molding shrinkage.
PC has excellent mechanical properties, good toughness, and a high glass transition temperature, but it has poor flowability and aging resistance. Blending PET with PC can improve impact strength. The blend of PET and PC has already been industrialized abroad and is widely used in automotive parts.
7. Elastomer Toughened PET
ABS is one of the most widely used polymers, not only having good toughness but also better comprehensive performance than HIPS. Blending PET with ABS can improve the impact strength of PET.
It was found in the research that the molecular weight of PET in the blend is very sensitive to processing temperature. The hydrolysis of the PET chain is related to the residual catalytic impurities in ABS. The reduction in the molecular weight of PET leads to a significant loss in impact performance and elongation at break, while it does not affect the modulus and flexural strength.
8. Advantages of USEON Twin-screw Extruder in PET Modification:

1. Efficient Mixing and Dispersion: The USEON co-rotating twin-screw extruder features high torque and excellent mixing and dispersing capabilities, ensuring uniform dispersion of various additives, fillers, and reinforcing materials into the PET matrix, thereby enhancing material uniformity and performance.
2. Precise Temperature Control: Equipped with a temperature control system from European suppliers, the USEON co-rotating twin-screw extruder allows precise control of processing temperatures, ensuring the material is processed at the optimal temperature and preventing degradation.
3. High Flexibility: The modular design of the USEON co-rotating twin-screw extruder allows flexible combination and adjustment of screw elements to meet the processing requirements of different materials, offering strong adaptability to various processes.
4. Self-cleaning Function: The USEON co-rotating twin-screw extruder has a good self-cleaning capability, reducing downtime for cleaning and improving production efficiency.
Part 5: PET Recycling
1. Chemical Recycling of PET
The synthesis of PET initially involves the condensation reaction of purified terephthalic acid (PTA) with ethylene glycol (EG) or the esterification reaction of dimethyl terephthalate (DMT) with EG, resulting in the product bis(2-hydroxyethyl) terephthalate (BHET), which subsequently undergoes polycondensation between diesters.
The essence of chemical recycling of PET bottles is the depolymerization reaction of PET, which completely degrades PET into monomers, such as PTA, DMT, BHET, EG, or partially into oligomers, under certain conditions. Common chemicals used for PET depolymerization include water (hydrolysis), methanol (alcoholysis), and EG (glycolysis).
Advantages:
- PET waste can be decomposed into monomers and/or oligomers and other chemicals.
- Compared with other recycling methods, it fully adheres to the principle of “sustainability.”
- The molecular weight (intrinsic viscosity) of recycled PET waste is preserved.
- Contaminated and highly complex waste streams can be recycled into products meeting required specifications.
- In the chemical recycling process of PET, the processability of the final products, such as printability and dyeability, can meet expected levels.
- No yellowing occurs in PET products during the chemical recycling process.
Disadvantages:
- The production cost of chemical depolymerization of PET is higher than that of directly producing PET, so without economic incentives, the cost of chemically recycled PET is higher.
- Large-scale production is required to make it cost-effective and feasible.
2. Physical Recycling of PET
Physical recycling refers to the process of converting waste PET products into deeply cleaned PET flakes through crushing, sorting, washing, and drying. These flakes are then plasticized and pelletized to become PET chips, which are thermoplastically molded into recycled PET bottles.
The intrinsic viscosity (IV) value required for PET blow molding should not be lower than 0.74 dl/g. However, the quality of post-consumer PET bottles decreases during the recycling process, and the intrinsic viscosity often drops during the melt extrusion process. This usually requires solid-phase polycondensation or liquid-phase adhesion to increase the molecular weight of PET to meet the blow molding requirements.
Advantages:
- Simple processing technology; low investment cost.
- Minimal negative impact on the environment.
Disadvantages:
- The complexity of contaminants hinders the physical recycling of PET.
- The discoloration and deterioration of product performance of PET waste during each recycling cycle are major issues in physical recycling.
- The production of cyclic and linear oligomers during the physical recycling process may reduce the printability and dyeability of the final product.
- Physical recycled PET tends to yellow due to intramolecular cross-linking and oxidation reactions.
Currently, the production technology for physical recycling mainly consists of degassing extrusion and adhesion of PET bottle materials, producing super-clean recycled PET flakes. The cleanliness largely depends on processing parameters such as temperature, pressure, airflow, and residence time during the process.
As a practitioner of sustainable development, Useon has been at the forefront of developing circular solutions for decades. Currently, Useon is the first company in China to recycle PET products using twin-screw and non-crystallization drying technology. After extensive practice, the recycling of new materials ensures that the viscosity remains below 0.03 dl/g, achieving primary recycling of PET waste.

As of June 2024, USEON has successfully deployed 130 PET recycling and pelletizing production lines for global customers, involving various PET recycling solutions such as bottle flakes, waste fabrics, yarns, films, and sheets. Among these, the bottle-to-bottle technology can recycle 2 million tons of drinking bottles annually, saving 70 million kilowatt-hours of electricity.
FAQs
PET has high transparency, good mechanical properties, chemical resistance, and electrical insulation. It has good heat and cold resistance but is relatively weak in impact resistance.
PET is a recyclable material, and recycling can reduce its environmental impact. However, if not properly managed, it can also become a source of microplastic pollution in the environment.
PET can be continuously used at temperatures up to 120°C, and it can withstand short-term temperatures of up to 65°C and cold temperatures down to -70°C.
Yes, PET is considered a safe packaging material and can be used for food and beverage packaging. It meets food safety standards and does not release harmful substances.
The recycling code for PET is "1", which is the identification number in the plastic recycling identification system used to mark containers made of recyclable PET material.




